Fluorescence of a substance is seen when the molecule is exposed to a specific wavelength of light (excitation wavelength or spectrum) and the light it emits ( the emission wavelength or spectrum) is always of a higher wavelength. To view this fluorescence in the microscope, several light filtering components are needed. Specific filters are needed to isolate the excitation and emission wavelengths of a fluorochrome. A bright light source with proper wavelengths for excitation is also needed. For normal fluorescence applications, this is a mercury vapor arc burner. For fluorescence confocal microscope applications where up to 95% of the emission light is filtered out, specific wavelength lasers are used as these are extremely bright.
Mercury arc burners are very bright lamps with a limited lifetime and require some maintenance and care to make sure that they are producing the brightest possible light beam for fluorescence excitation. For a discussion of the care of the mercury arc lamp, please CLICK HERE.
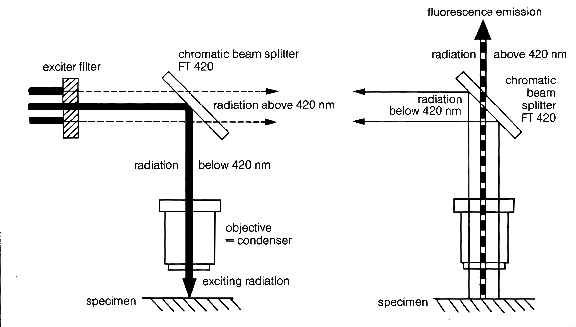
One other component is required: a dichroic beam splitter or partial mirror which reflects lower wavelengths of light and allows higher wavelengths to pass. A beam splitter is required because the objective acts as a condenser lens for the excitation wavelength as well as the objective lens for emission. One only wishes to see the light emitted from the fluorochrome and not any of the excitation light, and the beam splitter isolates the emitted light from the excitation wavelength. This epi-illumination type of light path is required to create a dark background so that the fluorescence can be easily seen. The wavelength at which a beam splitter allows the higher wavelengths to pass must be set between the excitation and emission wavelengths of any given fluorochrome so that excitation light is reflected and emission light is allowed to pass through it.
A typical fluorescence filter setup is shown in the diagram below (Courtesy of Carl Zeiss Inc., Germany).

Filter sets must be made to correspond to the excitation and emission characteristics of a give fluorochrome. Some sets are made to allow visualization of two or even three fluorochromes simultaneously.
Scientific personnel at UCLA may obtain a two page list of fluorochromes with excitation and emission wavelengths from the Facility (see Matt Schibler) or you can link to Carl Zeiss, Germany, to look at one.
The filter sets available on the Zeiss LSM 310 microscope in the Facility are:
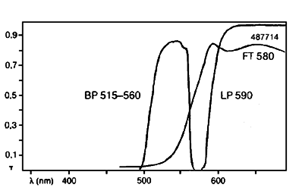
Zeiss Filterset 15: Green excitation for rhodamine (TRITC). (Propidium iodide (PI), Texas Red and some other red dyes may also be used, but the filter set is not optimal for these dyes).
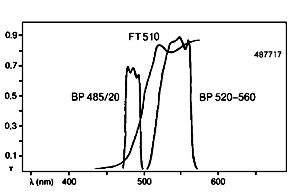
Zeiss Filterset 17: Blue excitation for fluorescein isothiocyanate (FITC). This filter set may be used for green fluorescent protein (GFP) but it is not optimal.
Here are some websites which show spectra of some available fluorescence sets and fluorochrome lists: